

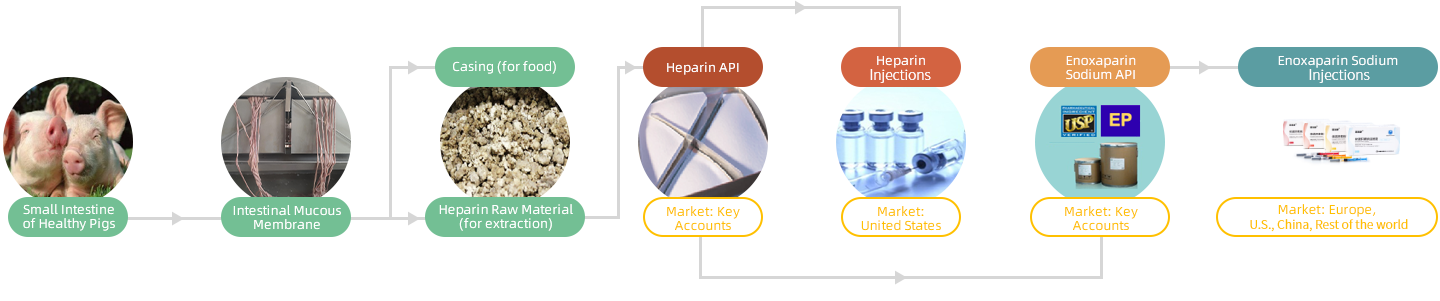
Techdow has a fully integrated global operation covering the entire heparin industrial chain, from the supply of raw materials and the manufacturing of APIs, to the scaled manufacturing of enoxaparin preparations. Techdow is among the few Chinese biopharmaceutical companies with a globally integrated close-loop operation in all parts of the heparin industrial chain. Techdow's integrated supply chain management system, designed to deliver traceability, quality, and reliable supply of raw materials, can ensure high product yield and quality.
Slide left and right to view the picture

1 drug adopted for treatment in
4 therapeutic areas
Prophylaxis of VTE in hip arthroplasty
Prophylaxis of VTE in knee arthroplasty
Prophylaxis of VTE in total hip arthroplasty (THA)
Prophylaxis of VTE in orthopedic trauma
Prophylaxis of VTE in pelvic surgery
Prophylaxis of VTE in general surgery
Prophylaxis of VTE in medical treatment
DVT anticoagulant therapy with or without pulmonary embolism
Extended treatment of DVT and pulmonary embolism (PE) and prevention of its recurrence in patients with active cancer
Anticoagulant in the treatment of unstable angina / non-Q wave myocardial infarction
Anticoagulant in PCI treatment
Anticoagulant in the medical treatment of STEMI
Anticoagulant in PCI treatment
Prevention of thrombosis in the extra-corporeal circulation of hemodialysis
7B3ACDFDEE4C400B9934A8EA749CDF04.png)
Patients with severe COVID-19 infections and those hospitalized for the infection
Patients with acute diseases including impaired respiratory functions or severe infections
Patients with deep venous thrombosis or pulmonary embolism (PE), excluding those PE patients who may need thrombolytic therapy or surgical treatment
High thrombotic risk patients receiving high-risk endoscopic surgeries
Acute lymphocytic leukemia
Cancer patients with thrombocytopenia
Rare hereditary hemorrhagic diseases
Unstable angina
Non-ST-elevated myocardial infarction
Acute ST-elevated myocardial infarction
Acute heart failure patients on medical treatment
Hospitalized patients with atrial fibrillation
ICU-treated COVID-19 infected patients with underlying heart diseases or prior cardiovascular events
Pregnant women with heart diseases
Pregnant women with heart diseases
Potential Applications* :
- Recurrent spontaneous abortion
- Antiphospholipid syndrome
- Prethrombotic state
Patients with brain tumors
Transient ischemic attacks
Patients with mid/high VTE risks receiving:
- Orthopedic surgery
- General surgical treatment
- Surgical cancer treatment
Cancer patients with venous thromboembolism (VTE)
Cancer patients with thrombocytopenia
Patients awaiting or received liver transplants
Chronic kidney disease patients with VTE
Extracorporeal circulation during dialysis
Rheumatic disease patients with VTE