

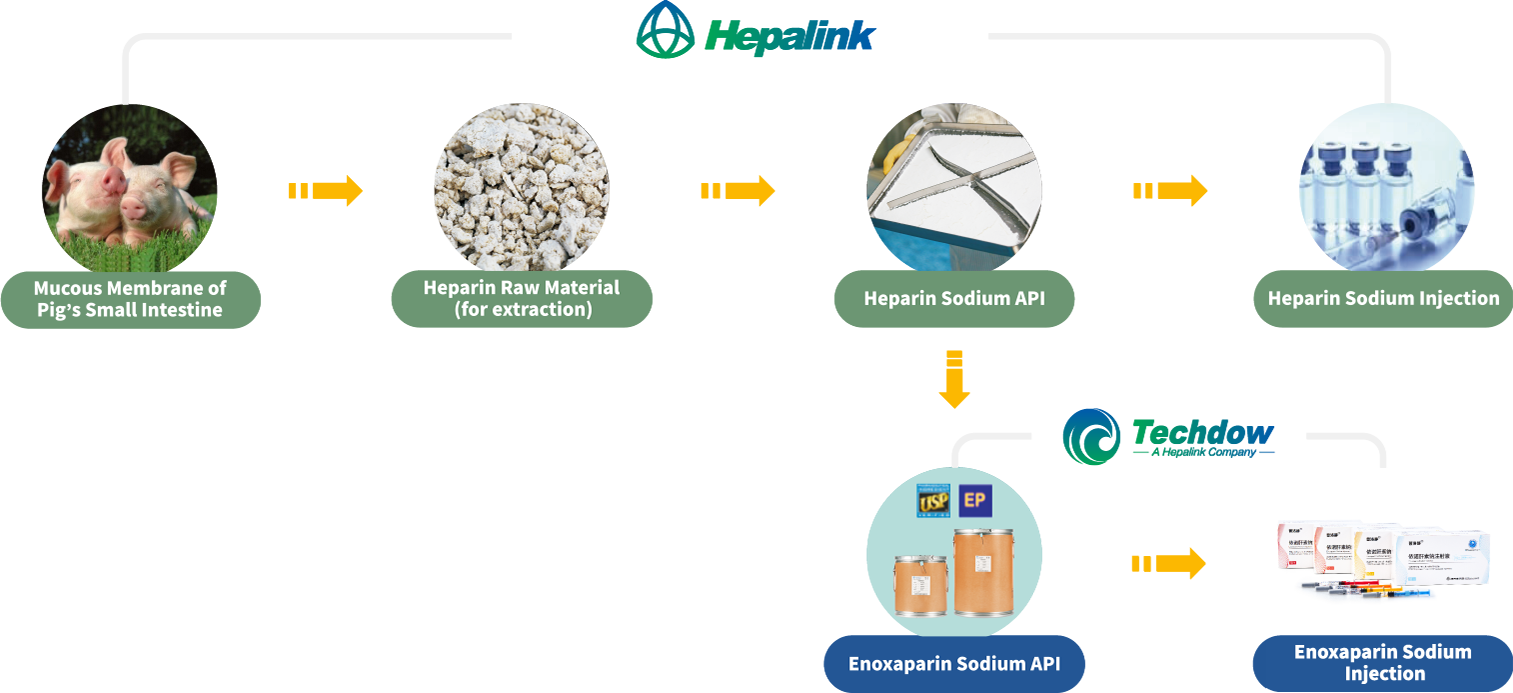
Leveraging Hepalink Group’s support and network, Techdow has built a fully-integrated global operation covering the entire heparin industrial chain, enabling effective control on raw materials and costs.
Slide left and right to view the picture
To ensure the quality of its raw material, Techdow only uses Hepalink’s heparin sodium as the API for its enoxaparin sodium products

Hepalink’s heparin sodium product has received 5 clean bills of health from inspections by the U.S. FDA
Hepalink’s heparin sodium product has been granted the Certificate of Suitability to the monographs of the European Pharmacopoeia (CEP)
In 2009, Hepalink participated in the amendment of the United States Pharmacopeia’s monograph on heparin sodium. The amended United States Pharmacopeia was published and took effect on Oct 1, 2009

Techdow’s enoxaparin sodium product received 2 clean bills of health from inspections by the U.S. FDA
Techdow has established long-term partnerships with numerous customers including leading multinational pharmaceutical companies